Abstract
Background
GATA2 is a zinc finger transcription factor that plays a critical role in lymphatic development and hematopoiesis. Germline mutations in GATA2 are highly penetrant, resulting in an autosomal dominant spectrum of disorders characterized by familial MDS (myelodysplastic syndrome)/ AML (acute myeloid leukemia), B/NK/dendritic cell immunodeficiency, lymphedema, and HPV-driven warts (Mir, Cancer Medicine, 2015). Myeloid clonal evolution is seen in approximately 75% of individuals with GATA2 haploinsufficiency and is associated with the acquisition of somatic mutations. We conducted this study to assess clinical correlates and outcomes of bone marrow (BM) failure and myeloid clonal evolution in patients with germline GATA2 haploinsufficiency .
Methods
Successive patients with germline GATA2 haploinsufficiency were identified from our institutional database. GATA2 mutations and deletions were confirmed using next generation (NGS) and Sanger sequencing (Mir, Cancer Medicine 2015). Time to clonal evolution was calculated from the time of earliest reported GATA2 -related clinical manifestation, to the development of clonal cytopenias of undetermined significance (CCUS), BM failure, or frank MDS/AML. In most patients, a myeloid relevant NGS panel was performed at the time of clonal evolution to identify the acquisition of somatic mutations. GVHD-free, relapse-free survival (GRFS) was calculated for hematopoietic stem cell transplant (HSCT) recipients (Holtan, Blood, 2015). Standard statistical measures were utilized.
Clinical Features:
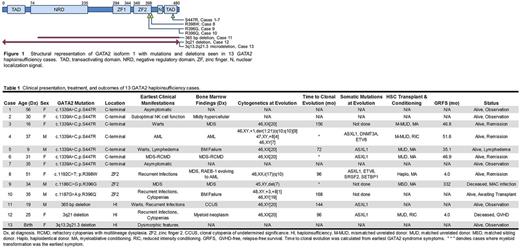
Thirteen patients with GATA2 haploinsufficiency were identified, median age 31 years (range, 0.5 to 57), of which 39% (5) were male. Median follow-up time was 46.1 months. Seven individuals comprised a kinship with a c.1339A>C, p.S447R mutation in the C-terminal domain of GATA2 . Three individuals had mutations within the zinc-finger 2 (ZF2) domain, specifically c.1192C>T, p.R398W, c.1186C>G, p.R396G, and c.1187G>A, p.R396Q, which interfere with DNA binding (Chong, Leukemia, 2017). Three individuals had larger deletions of chromosome 3q involving GATA2 (Table 1, Figure 1). Of the 13 individuals, two were asymptomatic and without clinical manifestations (Table 1). The remaining individuals' earliest symptoms included recurrent infections or immunodeficiency (n=5), warts (n=3), and cytopenias (n=2), with 3 cases of MDS/AML at the time of diagnosis.
Myeloid Clonal Evolution:
Nine (69%) of 13 patients developed myeloid clonal evolution; CCUS -1, bone marrow failure - 2, MDS- 3, JAK2/CALR/MPL negative, accelerated myeloproliferation- 1, and AML - 2. Of the cases that did not present with clonal evolution, the median time to clonal evolution was approximately 144 months (range, 96 to 168) from the earliest manifestations of GATA2 haploinsufficiency. At the time of clonal evolution, NGS was performed in 6 out of 9 patients, with all 6 (100%) demonstrating truncating ASXL1 mutations. In the 2 AML patients, additional somatic mutations included; DNMT3A, ETV6, SRSF2, and SETBP1 . Karyotypic abnormalities were seen in 4 (44%) of 9 cases (table 1).
Transplant Outcomes:
Seven patients underwent allogeneic HSCT for MDS, AML, or refractory immunodeficiency; 5 with myeloablative and 2 with reduced-intensity conditioning. Two (29%) were from 9/10 HLA mismatched unrelated donors (M-MUD), 3 (43%) were from MUD, and 1 (14%) each was from a matched related donor and a haploidentical source, respectively. 86% developed acute GVHD, mostly grade I-II, with the exception of one patient who developed grade 4 GI GVHD resulting in death. Transplant outcomes were favorable, with a GFRS of 88.3% at one year, and a 3 year relapse free survival of 66.7%. The 6 patients who have not undergone HSCT are alive and are being closely observed, or are in the planning phase for HSCT (Table 1).
Conclusions
Germline GATA2 haploinsufficiency is characterized by variable B/NK/dendritic cell immunodeficiency with an enhanced predisposition to myeloid neoplasms. Myeloid clonal evolution is seen >70% of cases, with almost all patients acquiring somatic truncating ASXL1 mutations at the time of evolution. In patients with AML, additional epigenetic regulator mutations are often seen. In our series, early allogeneic stem cell transplantation was associated with favorable outcomes.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal